Phytocannabinoids such as THC and CBD have been getting much attention in recent years—and rightly so. Yet the therapeutic benefits of medical cannabis extend far beyond merely external factors. Instead, these benefits are derived primarily from how phytocannabinoids positively influence the body’s internal cannabinoids and their integrated cellular receptors.
In other words, the relationship between phytocannabinoids and endocannabinoids is truly symbiotic. THC promotes relaxation via its activation of the brain’s CB1 receptors, for example, while CBD helps endocannabinoids themselves function their best.
So perhaps it’s only fitting that research developments in both these areas have also built upon each other over time. Phytocannabinoid research and endocannabinoid research are closely intertwined, after all.
Using chronological developments as our guide, let’s take a look at the last 80 years of medical cannabis research.
Early Research: Difficult, but Diligent
Circa 1930s: Scientists had already spent decades trying to identify what made cannabis work, but it was in the 1930s that research really intensified. Prior to this time, it was assumed that cannabis oil was a single chemical substance, but further analysis cleared that up.
Still, the process of discovery was restricted by limited technology. Having been spurred on by an in-vogue scientific interest in plant compounds (even cocaine and morphine were purified for ‘medicinal’ use), though, research persisted.
The only way to elucidate a molecule’s structure back then was to slowly degrade it until something recognizable formed. In this case, the heat-induced breakdown of cannabis oil yielded nearly pure CBN. With this finding, various laboratories began the process of trying to reverse-engineer CBN’s structure.
In 1940 in the US, Roger Adams and his team succeeded in lab synthesis of CBN. Adams was an award-winning chemist who found himself studying cannabis at the very start of its prohibition, but he pushed through this tension en route to publishing 27 cannabis-related studies. He even developed an “Adam’s score” to test the potency of cannabinoids that’s still in use today.

As impressive as these findings were, Adams didn’t actually ever identify CBN or CBD in its native plant form (though he did patent a technique for CBD’s isolation in 1940). Full elucidation would have to wait until several decades later; in the meantime, animal research with CBN showed it to be very sedating at high doses.
In the early 1960s, a young Israeli researcher named Dr. Raphael Mechoulam began his own research. A “natural products” chemist, Mechoulam related that he “was surprised to find out that while morphine had been isolated from opium 150 years previously, and cocaine had been isolated 100 years previously, the chemistry of cannabis was not well known.”
1963 & 1964: CBD Discovered, THC Follows
Backed by a brilliant team, Mechoulam set out to change that. In 1963 a then-revolutionary Nuclear Magnetic Resonance (NMR) spectroscope was used to fully identify the structure of CBD. With that, what Roger Adams had battled against technological limitations to do was finally accomplished.
The next year Mechoulam and the rest of his research team discovered delta-9 THC. Their published research described using hexane to extract and isolate individual components out of hashish—apparently, it worked.
Over the next decade, one minor cannabinoid after another was discovered. Mechoulam and his team identified many of them, and all except THC were found to be non-psychotropic. Animal studies followed, with THC (and its psychiatric impacts) naturally getting the most attention. Cannabinoids’ acid forms were thoroughly studied, too.
Throughout the 80s, various groups of researchers tried experimenting with synthetic cannabinoids. Unfortunately, this experimentation didn’t yield positive results. It turns out that nature really does know best; THC’s synthetic stereoisomers, on the other hand, came with all sorts of harsh side effects. A “very potent synthetic cannabinoid” called HU-210 was found to be “several thousand times more active than its synthetic mirror image (HU- 211)”.
The Search for Answers
By the late 80s, the list of health benefits associated with medical cannabis and its cannabinoids had grown pretty long. Researchers knew that the plant worked for epilepsy, neuropathy, and even cancer. These findings inspired countless people to try cannabis for themselves; in fact, the same 1975 THC & cancer study listed above later led Rick Simpson to produce his signature cannabis oil.
But nobody knew how cannabinoids worked — or what they were actually binding to — yet. As Mechoulam related at a recent European Congress on Epileptology:
“There was no conception [of endocannabinoids]. The mechanism of THC action was not known. People thought it had a general effect, so it was thought that the cannabinoids, particularly THC, do not act through a specific mechanism. The theory was that THC solubilizes in the cell membrane or something of that sort. It turns out that was wrong.”
To a scientist’s intuition, this was suspect. In a comprehensive historical overview, fellow researcher Lumír Hanuš confirms the thoughts of researchers at the time: “it was quite unacceptable to most neuroscientists that the brain will waste its resources to synthesize a receptor in order to bind a constituent of a plant.”
In other words, the working theory was that plant cannabinoids must be sharing their mode of action with something else. Somehow, somewhere, a receptor that just so happened to also accept cannabinoids existed.
Endocannabinoids: A Missing Piece of the Puzzle
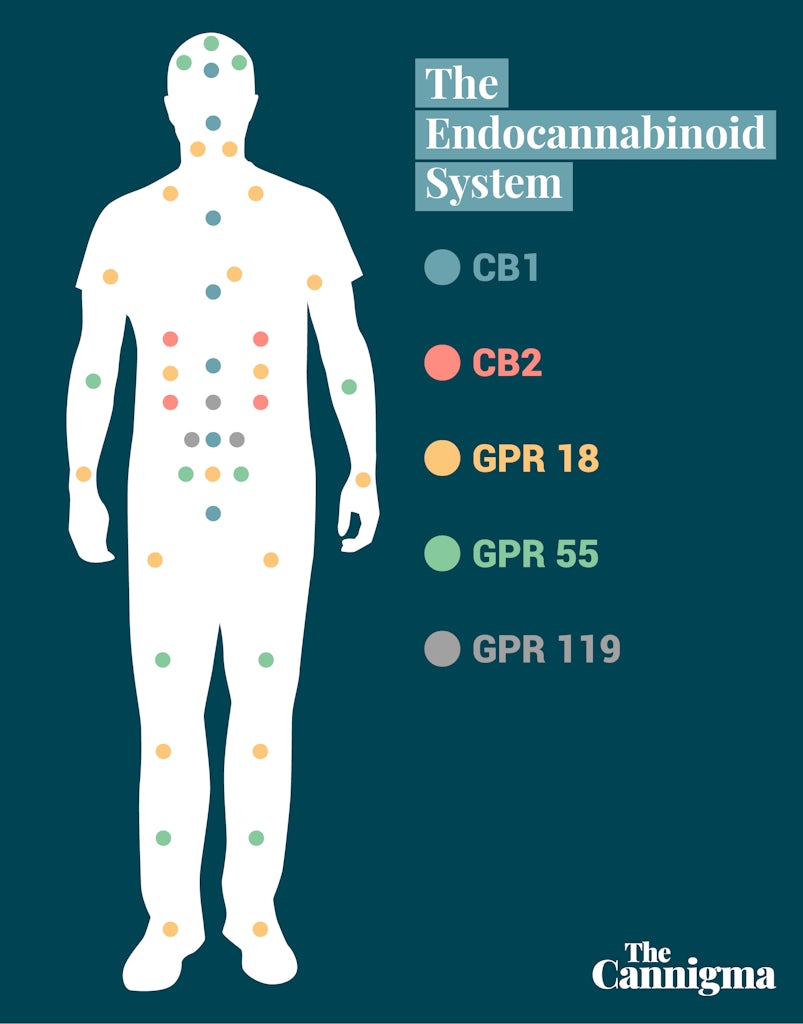
In 1988, it was discovered: a receptor in the brain that reacts to THC. Scientists named it cannabinoid receptor type-one (CB1 for short). Further research determined that CB1 receptors were most concentrated within the central nervous system; in fact, CB1 is actually the most commonly expressed brain receptor in its GPCR (G-protein-coupled receptor) class.
Shortly after, a second cannabinoid receptor was found. Naturally named CB2, this one wasn’t as active within the brain and didn’t seem to be fully agonized by THC. Instead, CB2 receptors were present in the peripheral body and its organ systems.
With this knowledge in mind, only one part of the puzzle remained: what else might be activating CB1 & CB2 receptors? Surely it wasn’t just plant cannabinoids. In other words, scientists deduced that there must be some that some sort of internal agonist keeping CB receptors activated—even when cannabis wasn’t. Back to Hanuš’s commentary again:
“The only reasonable [assumption] which could be made was that the brain must produce a neuronal mediator…a specific compound (or a family of compounds) which binds to and activates the cannabinoid receptor.”
The intuition of Hanuš and other chemists proved correct. Working together with American pharmacologist William Devane, Lumír Hanuš himself discovered the very first endocannabinoid: anandamide. A creative combination of the prefix ananda (Sanskrit for “bliss”) and the scientifically-inclined suffix -amide, anandamide’s discovery took the scientific world by storm. Now knowing what to look for, researchers soon identified several other fatty-acid based molecules that seemed to act on CB1 receptors in the brain. The primary one was named 2-arachidonoylglycerol, or 2-AG for short.
With the discovery of anandamide a biological puzzle finally came together: humans were affected by cannabis because they had a fully functional, three-part endocannabinoid system. Though this metaphorical puzzle is far from being fully solved, progress is being made. And with an increased understanding of the ECS came new and novel techniques to active for its activation. According to a 2005 study, the potential therapeutic targets of the ECS were found to include:
- Activation of CB2 receptors, or CB1 receptors + conjunctive opioids, for pain relief
- Activation of CB1 and CB2 without crossing the blood-brain barrier
- Activation of CB1 and CB2 by application of cannabinoids directly to the skin
- Activation of the “autoprotective” qualities of the ECS
- Activation of other systems with CBD, which does not directly activate CB1 or CB2
A New Century with New Theories
Cannabis research took a turn for the holistic at the turn of the century. Systematic thinkers like Dr. Ethan Russo and Dr. Bob Melamede used scientific findings to fuel theories about cannabis, life, and wellness; though unproven, these concepts rang true for countless medical cannabis patients in real life.
Dr. Russo came into the spotlight in 2004, when he introduced a concept called clinical endocannabinoid deficiency syndrome to the scientific world. Recounting his logic with Project CBD, Russo wondered:
“[…] What would a deficiency of endocannabinoid function look like? Well, we already knew that. If you don’t have enough endocannabinoids you have pain where there shouldn’t be pain. You would be sick, meaning nauseated. You would have a lowered seizure threshold. And just a whole litany of other problems.”
Left unchecked, endocannabinoid deficiencies can worsen to cause clinical diseases:
“a number of very common diseases seem to fit a pattern that would be consistent with an endocannabinoid deficiency, specifically these are migraine, irritable bowel syndrome, and fibromyalgia. […] They’re all hyper-algesic syndromes, meaning that there seems to be pain out of proportion to what should be going on, in other words you can look at the tissues they look okay, but there’s biochemically something that’s driving the pain.”
That something, in this case, is most likely a dysfunctional endocannabinoid system. Supplementing with cannabinoids like THC and CBD may start you on the course to correcting the deficiency, especially within the framework of other helpful lifestyle changes. In addition to his research into CECDS, Dr. Russo has been a vocal advocate of the entourage effect and its pharmacological importance.
In a more recent study called Cannabinoids in Health and Disease, Mechoulam and fellow research chemist Natalya Kogan declared that the therapeutic value of cannabinoids is too high to be put aside. Among other things, they noted, diseases such as emesis, epilepsy, osteoporosis, cancer, cardiovascular diseases, and obesity may be prime candidates for treatment with medical cannabis.
While Russo and Mechoulam have stayed on the conservative side of cannabis research, Dr. Bob Melamede’s extensive theories have made him more than a little controversial. As a molecular biologist specializing in DNA, RNA, and free radicals (he calls them “the friction of life”), Melamede came to see cannabinoids as a sort of universal antidote that reduces chemical friction.
“All biochemical change, good or bad, is stress. And the way we handle stress is with our cannabinoid receptor system, because it’s what allowed our brain to develop,”
The medical community (at least in the US) has largely disagreed, but medical cannabis users have found Dr. Bob’s theories useful—if not lifesaving.
The Floodgates Open on CBD Research
In recent years CBD research has taken center stage. Though once thought to be inert because its effects were so subtle (early researchers like Dr. Walter S. Loewe referred to CBD as a “relatively inactive component”), times have definitely changed. Legislative landmarks like the passing of 2014 and 2018’s Federal Farm Bills in the US have sparked an almost unprecedented interest in all things CBD and hemp.
With this interest has come more research — research that has made it abundantly clear that CBD is actually very active. It doesn’t directly agonize endocannabinoid receptors, though; instead CBD allosterically (i.e., indirectly) helps THC and endocannabinoids bind to their receptors.
Though cannabis has been used medicinally for at least 6,000 years, it’s only in the last hundred or so that the plant has been scientifically researched. As promising as the existing research is, this disparity in timelines means it’s possible that scientists are still missing something.
That said, the future of cannabis research seems headed in the right direction. Research on cannabinoids and the endocannabinoid system seems to build with each passing day, and thankfully much of this research comprises pro-whole plant, pro-entourage effect findings.
Perhaps Lumír Hanuš said it best in his interview with Project CBD:
“I can see two different directions [for the future of cannabis research]. One will focus on production of derivatives of active compounds that could be patented and sold in order to make as much money as possible. The other direction puts patients before profit and focuses on the use of the whole plant and synergistic effects of its compounds.”
In the future, look for further research into this other direction, research on the entourage effect and its mysteries. The nature of cannabis’s diversity and complexity means there’s still much to be discovered.
For example, research has already found that THC and CBD synergize. Researchers have also found that terpenes like myrcene help open up the blood-brain barrier to accompanying cannabinoids. Another study, this one by Mechoulam et al, has detailed how whole plant extracts are superior to CBD isolate. But elucidating exactly what makes the combined effect of each and every plant compound in cannabis will take much, much more research. Thankfully, medical cannabis research pioneers are committed to the cause.
Sign up for bi-weekly updates, packed full of cannabis education, recipes, and tips. Your inbox will love it.

 Shop
Shop Support
Support