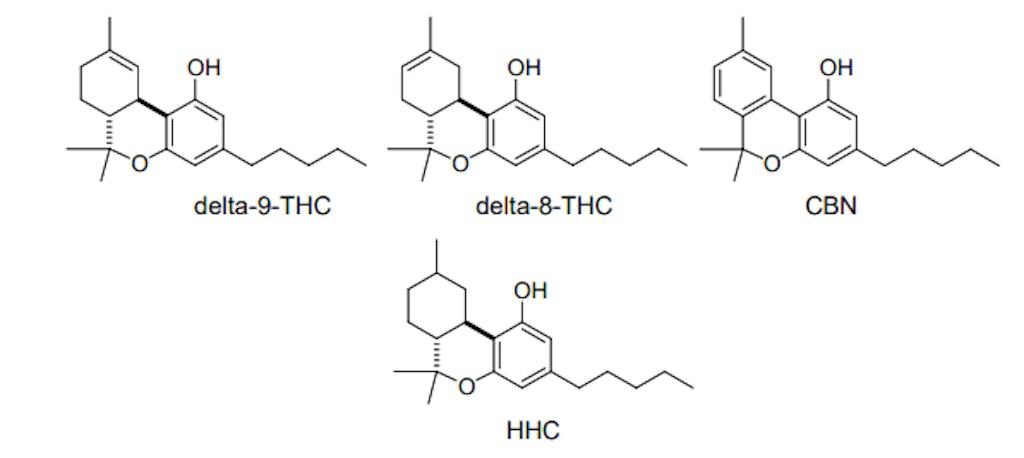
Hexahydrocannabinol (HHC), which was patented in the late 1940s as a “therapeutic substance having marihuana activity,” is a cannabinoid that’s growing in popularity and availability. This synthetic derivative of naturally occurring delta-9-Tetrahydrocannabinol (delta-9-THC) was first reported by noted cannabis chemist Professor Roger Adams at the University of Illinois in 1940. 1 2
HHC was synthesized in Adams’ laboratory as part of research to understand the active compounds in the cannabis plant. It was also later found that HHC can be produced in the lab during the conversion of THC to CBN in what is known as a disproportionation reaction. The importance of this finding is that, similar to CBN, HHC may occur naturally as a degradation product in aged cannabis, extracts, and concentrates. 3
What is hydrogenation?
Hydrogenation is a well-known industrial process where hydrogen (H2) is added to organic compounds that possess unsaturation (i.e., double bonds) to generate the corresponding saturated compound. The resulting saturated product has enhanced oxidative stability vs. the unsaturated starting material from which it was derived. An everyday example of this is the hydrogenation of unsaturated fats used as shortening for shelf-stable baked goods and margarine. 4
Hydrogenation of THC isomers such as delta-9-THC or delta-8-THC results in the formation of hexahydrocannabinol (HHC), a hydrogenated cannabinoid. As with most other cannabinoids, HHC can also be obtained from thermal decarboxylation of hexahydrocannabinolic acid (HHCA). This hydrogenated cannabinoid acid can be created through the hydrogenation of delta-9-tetrahydrocannabinolic acid (delta-9-THCA), the carboxylated precursor to delta-9-THC produced by cannabis. 5
What does HHC do in the body?
Due to its structural similarity with the known active compounds in cannabis, namely delta-9-THC and CBN, there has long been an interest in HHC as a biologically active compound. During the initial work by Adams in 1940, he pointed out that “the hexahydrocannabinol is also active.” Soon after its initial discovery by Adams, HHC was investigated by other research groups for its activity in behavioral observation models both in rabbits and dogs where psychoactivity similar to what would be expected of THC was observed. 6

In 1971, Raphael Mechoulam, the Israeli scientist who identified the chemical structure of THC, reported on the separation of the two HHC epimers (or forms) produced from THC hydrogenation. The research found that the beta-form of HHC was more active than alpha-form in a behavioral observation model in monkeys. He surmised that even though the differences in the molecules are minimal, this slight difference in 3-D orientation of beta-HHC makes it more active than the alpha form.7
More recently, Kim and co-workers have reported on novel hexahydrocannabinol-based compounds that have been investigated for their cancer-fighting properties in several preclinical models. The investigators employed an elegant catalytic one-pot method published by Lee and Xia, which can synthesize each of the HHC epimers independently and not as a mixture. 8 9
There is some reason to believe that these hydrogenated cannabinoids hold therapeutic promise. In a small experiment done on rodents with tumors known as glioblastomas, scientists found that hydrogenated cannabinoids performed as well or better than the non-hydrogenated counterparts at reducing tumor size. 10
HHC metabolism
In 1991, Harvey and Brown reported that, similar to THC, HHC is oxidized by liver microsomes from rats, mice, hamsters, guinea pigs and rabbits to form hydroxylated derivatives including 11-hydroxy-hexahydrocannabinol (11-OH-HHC) as the major metabolite identified. This would indicate that similar to THC, HHC is metabolized in humans to 11-hydroxy-HHC which could in part be responsible for the psychoactive effects of HHC when consumed. 11
Is HCC safe for humans?
There are no published studies on the efficacy or safety of HHC in human subjects but that has not stopped some manufacturers from producing and selling HHC products.
As far as effects of HHC, anecdotal evidence in human use points towards its uplifting, psychoactive properties when inhaled, likely due to CB1 agonism similar to THC.
Where is HHC available?

HHC is currently being produced as a purified distillate from CBD isolate. The CBD being used to produce HHC is derived from industrial hemp, which was legalized in the US via the 2018 Farm Bill. Additionally, HHC is currently not scheduled or listed in the Controlled Dangerous Substance Act. HHC is currently being manufactured for use in vape pens and edibles by industrial hemp processors who are producing CBD. There are even a few third-party testing labs that are developing analytical methods to test and validate HHC sample identity and purity.
It is reasonable to think that market demand for HHC and compounds like it will continue to increase, especially while federal prohibition remains. The development of analytical standards, regulations, more data from toxicology studies and clinical trials, as well as substantial consumer education will all be imperative to the development of the hydrogenated cannabinoid market.
Sources
- Adams et al J. Am. Chem Soc. 1940, Vol. 62, pp. 2402-2405.
- Adams US 2,419,936, Preparation of compounds with marihuana activity. May 1947
- Garrett ER, Gouyette AJ, Roseboom H. Stability of tetrahydrocannabinols II. J Pharm Sci. 1978;67(1):27-32. doi:10.1002/jps.2600670108
- Rylander, P. N.: Catalytic Hydrogenation in Organic Syntheses, New York: Academic Press, 1979
- Scialdone US 10, 071,127, Hydrogenation of cannabis oil. July 4, 2017.
- Todd et al J. Chem Soc. Part VII, 1941, pp. 169-172.
- Edery, H., Grunfeld, Y., Ben-Zvi, Z. and Mechoulam, R. (1971), Structural requirements for cannabinoid activity. Annals of the New York Academy of Sciences, 191: 40-53. https://doi.org/10.1111/j.1749-6632.1971.tb13985.x
- Thapa D, Lee JS, Heo SW, et al. Novel hexahydrocannabinol analogs as potential anti-cancer agents inhibit cell proliferation and tumor angiogenesis. Eur J Pharmacol. 2011;650(1):64-71. doi:10.1016/j.ejphar.2010.09.073
- Lee, Yong & Xia, Likai. (2008). Efficient one-pot synthetic approaches for cannabinoid analogues and their application to biologically interesting (-)-hexahydrocannabinol and (+)-hexahydrocannabinol. Tetrahedron Letters. 49. 3283-3287. 10.1016/j.tetlet.2008.03.075.
- Scialdone US 10, 071,127, Hydrogenation of cannabis oil July 4, 2017.
- J Harvey and N K Brown. In vitro metabolism of the equatorial C11-methyl isomer of hexahydrocannabinol in several mammalian species. Drug Metabolism and Disposition May 1, 1991, 19 (3) 714-716;
Sign up for bi-weekly updates, packed full of cannabis education, recipes, and tips. Your inbox will love it.

 Shop
Shop Support
Support